Answer:
3.01 x 10²³ molecules
Step-by-step explanation:
Given parameters:
Number of moles of oxygen atoms = 6.17moles
Unknown:
Number of molecule of tin(IV) nitrate = ?
Solution:
To solve this problem, let us write the formula of tin (IV) nitrate;
N₄O₁₂Sn
Now,
12 moles of Oxygen can be found in 1 mole of N₄O₁₂Sn
6.17 moles of oxygen will be found in
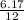 = 0.5mole of N₄O₁₂Sn
= 0.5mole of N₄O₁₂Sn
So;
1 mole of a substance contains 6.02 x 10²³ molecules
0.5 mole of N₄O₁₂Sn will contain 0.5 x 6.02 x 10²³ = 3.01 x 10²³ molecules