Answer:
123.1L
Step-by-step explanation:
Given parameters:
Molarity of Cu(NO₃)₂ = 0.650M
Mass of Cu(NO₃)₂ = 15g
Unknown:
Volume of Cu(NO₃)₂ = ?
Solution:
To solve this problem, we have to first find the number of moles of the given mass;
Number of moles of Cu(NO₃)₂ =

Molar mass of Cu(NO₃)₂ = 63.5 + 2 (14 + 3(16)) = 187.5g/mol
Number of moles of Cu(NO₃)₂ =
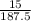 = 0.08 mol
= 0.08 mol
Molarity is given as;
Molarity =
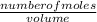
Volume =
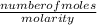
Insert the parameters and solve;
Volume =
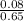 = 0.12L
= 0.12L
Now mL;
1L = 1000mL
0.12L will give 1000 x 0.12 = 123.1L