Answer:
Fe =52.2%, O = 44.9%, H = 2.81%
Step-by-step explanation:
Percentage composition is also known as percentage by mass.
First, find the Mr of the compound.
Mr of Fe(OH)₃ = 55.8 + (16 × 3) + (1 × 3)
= 106.8
Now Divide the mass of the element, as per the compound, by the Mr of the compound found and times that by 100.
% composition of Fe =
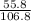 × 100 = 52.2%
× 100 = 52.2%
% composition of O =
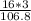 × 100 = 44.9%
× 100 = 44.9%
[There are 3 oxygen atoms in the compound Fe(OH)₃, so we will multiply the atomic mass of oxygen with the number of atoms in the compound: 16×3 ]
% composition of H =
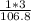 × 100 = 2.81%
× 100 = 2.81%
[There are 3 hydrogen atoms in the compound Fe(OH)₃, so we will multiply the atomic mass of hydrogen with the number of atoms in the compound: 1×3 ]