Answer:
3.2 x 10²¹molecules
Step-by-step explanation:
Given parameters:
Volume of nitrogen gas = 120cm³
Unknown:
Mass of nitrogen gas = ?
Number of molecules = ?
Solution:
To solve this problem, note that;
1 mole of gas occupies a volume of 22.4L at STP
Now;
convert 120cm³ to L ;
1000cm³ = 1L
120cm³ gives 0.12L
Since;
22.4L of gas has 1 mole at STP
0.12L of gas will have
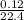 = 0.0054mole at STP
= 0.0054mole at STP
So;
Mass of N₂ = number of moles x molar mass
Molar mass of N₂ = 2(14) = 28g/mol
Mass of N₂ = 0.0054 x 28 = 0.15g
Now;
1 mole of a gas will have 6.02 x 10²³ molecules
0.0054 mole of N₂ will contain 0.0054 x 6.02 x 10²³ =
3.2 x 10²¹molecules