Answer:
2AlBr₃ + 3K₂SO₄ → 6KBr + Al₂(SO₄)₃
Step-by-step explanation:
Given species;
Reactants
Aluminum bromide = AlBr₃
Potassium sulfate = K₂SO₄
Products
Potassium bromide = KBr
Aluminum sulfate = Al₂(SO₄)₃
So, the reaction equation is given as:
AlBr₃ + K₂SO₄ → KBr + Al₂(SO₄)₃
To balance this chemical equation, let us an algebraic approach;
aAlBr₃ + bK₂SO₄ → cKBr + dAl₂(SO₄)₃
Conserving Al;
a = 2d
Conserving Br;
3a = c
Conserving K;
2b = c
Conserving S;
b = 3d
Conserving O;
4b = 12d
if a = 1;
c = 3
b =
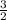
d =
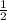
Now, multiply through by 2;
a = 2, b = 3, c = 6 and d = 1
2AlBr₃ + 3K₂SO₄ → 6KBr + Al₂(SO₄)₃