Answer: 0.02 mol
Step-by-step explanation:
The atomic mass of zinc is 65.409 g/mol, so 1.14 grams of zinc is about
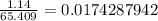 moles.
moles.
From the equation, we know that for every mole of zinc consumed, there is 1 mole of zinc(II) chloride produced, so about 0.02 moles can be prepared.