Answer:
- 8.5 °C
- 34 °C
- 51 °C
Step-by-step explanation:
The relation between temperature and volume at constant pressure is given by Charles Law.
Scenario 1 : Volume is halved
⇒ Let initial volume be V
⇒
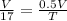
⇒ T = 17 × 0.5
⇒ T = 8.5 °C
Scenario 2 : Volume is doubled
⇒
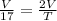
⇒ T = 17 × 2
⇒ T = 34 °C
Scenario 3 : Volume is tripled
⇒
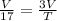
⇒ T = 17 × 3
⇒ T = 51 °C