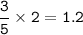Moles of Carbon dioxide : 1.2
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
Reaction(balanced)
C₃H₈ + 5O₂=> 3CO₂ + 4H₂O
mol of O₂ :
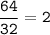
mol of CO₂ :