Answer:
B
Explanation:
Let's start by finding the y-intercept because it is easy. The Y-intercept is actually the point when x is 0. look at the graph, and find that when x is 0, y is 1. so the point (0,1) is the y-intercept. We say that the y-intercept is 1 in this case.
what about the slope? get any 2 points and use the slope formula.
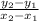
take the points (0,1) and (2,0). Use the equation and you get the slope of -1/2