Answer:
The largest wavelength of light that will produce photoelectrons from this surface is 324.6 nm.
Step-by-step explanation:
Given;
work function, Ф = 3.82 eV
The work function of the metal is minimum energy required to produce electron from the metal surface.
Ф = hf
where;
h is Planck's constant = 6.626 x 10⁻³⁴ J/s
f is the frequency of the photon
f = c / λ
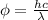
where;
c is speed of light = 2.998 x 10⁸ m/s
λ is the wavelength = ?
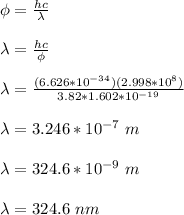
Therefore, the largest wavelength of light that will produce photoelectrons from this surface is 324.6 nm.