Answer:
1.327 g Ag₂CrO₄
Step-by-step explanation:
The reaction that takes place is:
- 2AgNO₃(aq) + K₂CrO₄(aq) → Ag₂CrO₄(s) + 2KNO₃(aq)
First we need to identify the limiting reactant:
We have:
- 0.20 M * 50.0 mL = 10 mmol of AgNO₃
- 0.10 M * 40.0 mL = 4 mmol of K₂CrO₄
If 4 mmol of K₂CrO₄ were to react completely, it would require (4*2) 8 mmol of AgNO₃. There's more than 8 mmol of AgNO₃ so AgNO₃ is the excess reactant. That makes K₂CrO₄ the limiting reactant.
Now we calculate the mass of Ag₂CrO₄ formed, using the limiting reactant:
- 4 mmol K₂CrO₄ *
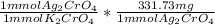 = 1326.92 mg Ag₂CrO₄
= 1326.92 mg Ag₂CrO₄
- 1326.92 mg / 1000 = 1.327 g Ag₂CrO₄