Answer:
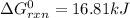
Step-by-step explanation:
Hello!
In this case, since the Gibbs free energy of any process is related with the enthalpy change, temperature and entropy change as shown below:
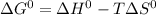
For a chemical reaction it is simply modified to:
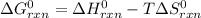
Thus, since the enthalpy of reaction is given as -304.2 kJ and the entropy as -414.2 J/K (-0.4142 kJ/K), at 775 K the Gibbs free energy of reaction turns out:
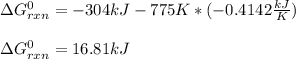
Whose result means this is a nonspontaneous reaction.
Best regards!