Answer:
The correct option is (a).
Step-by-step explanation:
Given that,
The energy of photon, E = 2.52 eV
We need to find the wavelength of the photon in nm. The formula for the energy of a photon is given by :
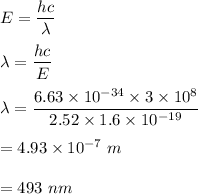
The nearest option is a) i.e. 492.34127 nm.