Answer:
the volume delivered by the pipette = 22.32 mL
Step-by-step explanation:
To calculate this, let us first note that the density of water relates it weight and its volume (density = mass ÷ volume), hence we are going to use density to determine the volume.
Density of water = mass/volume = 0.997 g/ mL
mass = 22.25g
Density = 0.997g/mL
volume = ?
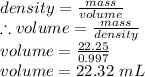
∴ the volume delivered by the pipette = 22.32 mL
Please note that this calculation is based on the fact that the weight of the empty flask has been determined and canceled out.