Answer:
The final pressure is 0.788 atm (option b).
Step-by-step explanation:
Boyle's law says that the volume occupied by a given gaseous mass at constant temperature is inversely proportional to pressure. That is: if the pressure increases, the volume decreases, while if the pressure decreases, the volume increases. This is expressed mathematically as the product of pressure times volume equal to a constant value:
P*V=k
Assuming a certain volume of gas V1 that is at a pressure P1 at the beginning of the experiment, by varying the volume of gas to a new value V2, then the pressure will change to P2, and it will be fulfilled:
P1*V1=P2*V2
In this case:
- P1= 2.14 atm
- V1= 3 L
- P2= ?
- V2= 8.15 L
Replacing:
2.14 atm*3 L= P2* 8.15 L
Solving:
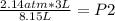
0.788 atm= P2
The final pressure is 0.788 atm (option b).