Answer:
c) removing fluoride ions.
Step-by-step explanation:
Hello!
In this case, for this solubility product problem, it is very convenient to write the undergoing ionization reaction:
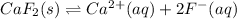
In such a way, via the Le Chatelier's principle, we remember that the addition of reactants shifts the reaction towards products, adding products shifts the reaction towards reactants, removing reactants shifts the reaction towards reactants and removing products shifts the reaction towards products. In means, that the best way to shift this reaction away from solid calcium fluoride and toward the dissolved ions is c) removing fluoride ions, because it is a product and its removal favors the formation of more products.
Best regards!