Answer:
11.5moles of oxygen gas
Step-by-step explanation:
The reaction equation is shown below;
C₂H₆O + 3O₂ → 2CO₂ + 3H₂O
We were given 11.5moles of H₂O produced;
Since the equation is balanced;
3 mole of oxygen gas was used to produce 3 mole of water
11.5 moles of water will be produced
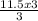 = 11.5moles of oxygen gas
= 11.5moles of oxygen gas