Answer:
Chlorine (35.44 amu)
Step-by-step explanation:
An unknown element is found to have three naturally occurring isotopes with atomic masses of 34.9689 (76.000%), 36.9659 (23.988%) and 35.9683 (0.012%).
We need to find the mass of the unknown element. When abundances is given, then atomic mass is given by :
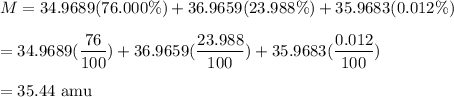
So, the atomic mass of the unknown element is 35.44 amu and it is chlorine.