Answer:
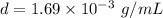
Step-by-step explanation:
Given that,
Mass, m = 0.186 g
Volume occupies, V = 110 mL
We need to find the density of Nitrogen gas. The mass per unit volume is called density of an object. It is given by the formula as follows :
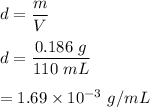
So, the required density is
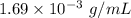 .
.