Answer:
Both
Step-by-step explanation:
The combined gas law is also known as the general gas law.
From the ideal gas law we assume that n = 1;
So;
PV = nRT
and then;
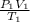 =
=
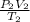
If we cross multiply;
P₁V₁T₂ = P₂V₂T₁
So;
T₁ =
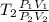
Also;
V₂ =
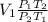
So from the choices both are correct