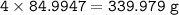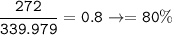Yield of reaction = 80%
Further explanation
Reaction
Cu(NO₃)₂ (aq) + Na₂S (aq) ⇒ 2 NaNO₃ (aq) + CuS (s)
mol Cu(NO₃)₂ :(MW 187,56 g/mol)
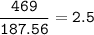
mol Na₂S :(MW 78,0452 g/mol)
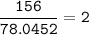
Limiting reactant⇒Na₂S( 2 < 2.5)
mol of NaNO₃ (MW = 84,9947 g/mol)
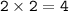
mass NaNO₃ (theoretical)