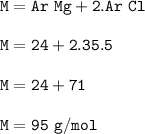Molar mass of MgCl₂ : 95 g/mol
Further explanation
An atomic mass unit ( amu or "u") is a relative atomic mass of 1/12 the mass of an atom of carbon-12.
The molar mass(molecular mass-formula mass) of a compound is the sum of the relative atomic mass (Ar) of the constituent elements of the compound
Can be formulated :
M AxBy = (x.Ar A + y. Ar B)
Compound MgCl₂