Answer:
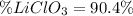
Step-by-step explanation:
Hello!
In this case, since the undergoing chemical reaction is:
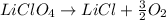
It is widely known that when a gas given off from a reaction is collected over water, we can compute its pressure by minusing the total pressure 762 mmHg and the vapor pressure of water at the experiment's temperature (20 °C) in this case 17.5 mmHg as shown below:
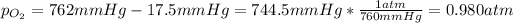
Next, by using the ideal gas equation we compute the yielded moles of oxygen considering the collected 313 mL (0.313 L):
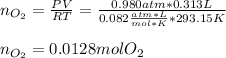
Now, via the 3/2:1 mole ratio between oxygen and lithium chlorate (molar mass = 90.39 g/mol), we compute the original mass of decomposed lithium chlorate as follows:
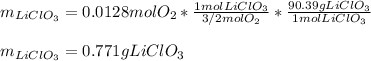
Now, the percentage is computed as shown below:
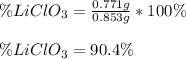
Best regards!