The maximum number of balloons that can be inflated with the available fuel and conditions is approximately 3.9.
How to find maximum number?
Balloon radius (r) = 110 cm (convert to m later)
Initial air temperature (
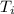 ) = 25°C + 273.15 K
) = 25°C + 273.15 K
= 298.15 K
Final air temperature (
 ) = 100°C + 273.15 K
) = 100°C + 273.15 K
= 373.15 K
Density of air at final temperature (
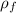 ) = 0.946 kg/m³
) = 0.946 kg/m³
Butane mass (
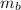 ) = 3.00 kg
) = 3.00 kg
Specific heat capacity of air (
 ) = 1.009 J/g°C (convert to J/kg°C later)
) = 1.009 J/g°C (convert to J/kg°C later)
Formation enthalpy of butane (
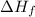 ) = -2878.4 kJ/mol
) = -2878.4 kJ/mol
Calculate balloon volume:
Volume of a sphere = (4/3)πr³
Balloon volume (
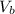 ) = (4/3)π × (1.1 m)³
) = (4/3)π × (1.1 m)³
≈ 5.24 m³
Calculate air mass required per balloon:
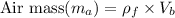
= 0.946 kg/m³ × 5.24 m³
≈ 4.95 kg
Calculate temperature difference required per balloon:
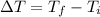
= 373.15 K - 298.15 K
= 75 K
Calculate heat required per balloon:
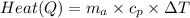 (convert
(convert
 to J/kg°C first)
to J/kg°C first)
Q = 4.95 kg × 1009 J/(kg°C) × 75 K
Q ≈ 371452.5 J
Calculate moles of butane burned per balloon:
Moles of butane (
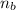 ) = Q / (
) = Q / (
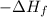 ) (convert
) (convert
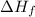 to J/mol first)
to J/mol first)
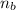 = 371452.5 J / (-2878400 J/mol)
= 371452.5 J / (-2878400 J/mol)
≈ 0.013 mol
Calculate number of balloons possible:
Total moles of butane (n_b_total) =
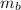 / (molar mass of butane)
/ (molar mass of butane)
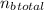 = 3.00 kg / 58.12 g/mol
= 3.00 kg / 58.12 g/mol
≈ 0.051 mol
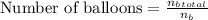
≈ 3.9
Therefore, the maximum number of balloons that can be inflated with the available fuel and conditions is approximately 3.9.