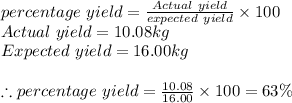Answer:
percentage yield = 63%
Step-by-step explanation:
The yield efficiency or percentage yield measure the amount of products that are formed from a given amount of reactant. For a percentage yield of 100, all the reactants are completely converted to product. Mathematically, the percentage yield is given by: