Answer:
The ionic compound NaCl has a Face Centered Cubic unit cell
The site for atoms are Face, center and Edges corner.
Effective number of atoms
Na+
1centers + 12edges x
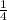 = 4 Na+
= 4 Na+
Cl-
6face x
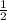 +
+
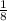 x8 corners = 4Cl-
x8 corners = 4Cl-
This is just one unit cell effective atom, there are thousand's of such unit cells are combined to form one such structure of NaCl. The real formula of NaCl is Na4Cl4 but for simplicity we use empirical formula that is NaCl.