Answer:
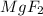
Step-by-step explanation:
Hello!
In this case, since, when we see an ionic binary salt like that formed by magnesium (metal) and fluorine (nonmetal), in order to set up the correct molecular formula we need to check out the periodic table in order to identify the suitable oxidation states, since metals remain positively charged as they lose electrons and nonmetals negatively charged as they gain electrons.
In such a way, since the oxidation state of magnesium is +2 and that of fluorine is -1 we write:
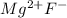
Next, we need to exchange the oxidation states as subscripts without the charge to obtain:
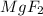
Which is the corrected molecular formula for magnesium fluoride.
Best regards!