Answer:
94.0 g.
Step-by-step explanation:
Hello!
In this case, since the lay of conservation of mass states that energy cannot be neither created nor destroyed, during a chemical reaction it is seen that the reactions undergo a change by which bonds can be broken or formed depending on the case. Thus, for the formation of sodium chloride we evidence the formation of the Na-Cl bond which means sodium is combined with chlorine according to the following chemical reaction:
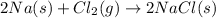
It means that the reuslting mass of product is:
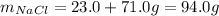
Best regards!