Answer:
Option (B) is correct.
Step-by-step explanation:
Given that the molecules of hydrogen gas (
 ) react with molecules of oxygen gas (
) react with molecules of oxygen gas (
 ) in a sealed reaction chamber to produce water (
) in a sealed reaction chamber to produce water (
 ).
).
The governing equation for the reaction is
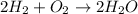
From the given, the only fact that can be observed that 2 moles of
 and 1 mole of
and 1 mole of
 reacts to produce 2 moles of
reacts to produce 2 moles of
 .
.
As the mass of 1 mole of
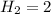 grams ... (i)
grams ... (i)
The mass of 1 mole of
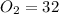 grams ...(ii)
grams ...(ii)
The mass of 1 mole of
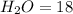 grams (iii)
grams (iii)
Now, the mass of the reactant = Mass of 2 moles of
 + mass 1 mole of
+ mass 1 mole of

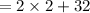 [ using equations (i) and (ii)]
[ using equations (i) and (ii)]
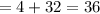 grams.
grams.
Mass of the product = Mass of 2 moles of

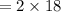 [ using equations (iii)]
[ using equations (iii)]
=36 grams
As the mass of reactants = mass of the product.
So, mass is conserved.
Hence, option (B) is correct.