The question is incomplete. Here is the complete question.
Two kg of a two phase liquid vapor mixture of carbon dioxide (CO₂) exists at -40°C in a 0.05m³ tank. Determine the quality of the mixture, if the values of specific volume for saturated liquid and saturated vapor CO₂ at -40°C are
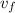 = 0.896 x 10⁻³m³/kg and
= 0.896 x 10⁻³m³/kg and
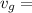 3.824 x 10⁻²m³/kg, respectively.
3.824 x 10⁻²m³/kg, respectively.
Answer: x = 1
Step-by-step explanation: In a phase change of a pure substance, at determined pressure and temperature, the substance exists in two different phases: saturated liquid and saturated vapor.
Quality (x) is the ratio of saturated vapor in the mixture and can be written as:
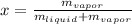
It has value between 0 and 1: x = 0 for saturated liquid and x = 1 for saturated vapor.
When related with volumes, quality is rearranged as:
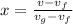
Solving for x:
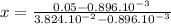
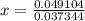
x = 1.3
Quality of mixture of carbon dioxide is x = 1, which means it's for saturated vapor.