Answer:
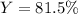
Step-by-step explanation:
Hello!
In this case, for the given chemical reaction, since it is started with 10.0 g of CO, we can compute the theoretically yielded grams of carbon dioxide given the 2:2 mole ratio between them, on the chemical reaction:
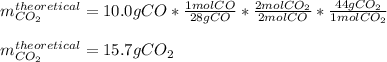
Next, since the percent yield measures how much of the product is actually yielded, we compute it as shown below:
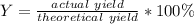
Whereas the theoretical yield is 12.81 g and the theoretical one that 15.7 g. Therefore, the percent yield turns out:
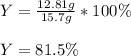
Regards!