Answer:
(C) Oxygen because it has the strongest attractive force and the largest
electronegativity
Step-by-step explanation:
Oxygen will be most reactive
configuration =
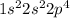
As oxygen need only two electrons to complete its octet.
Oxygen will have stronger force than nitrogen as it will tend to pull electrons more to complete its octet. Also along the period electronegativity increases so oxygen has higher electronegativity than nitrogen