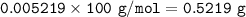The amount of CaCO₃ in the tablet : 0.5219 g
Further explanation
Reaction
1. CaCO₃(in antacid) + 2HCl⇒ CaCl₂+H₂CO₃
2. Titration ⇒ HCl+NaOH⇒NaCl+H₂O
For titration :
M₁V₁.n₁=M₂V₂n₂(n=acid/base valence⇒NaOH/HCl=1)
mol HCl=mol NaOH = excess HCl
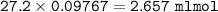
mol HCl

Moles of HCl used (reacted with CaCO₃) :
initial HCl - excess HCl =
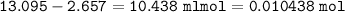
From reaction 1 :
mol HCl : mol CaCO₃ = 2 : 1
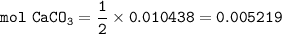
mass of CaCO₃ :