Answer:
3.3g
Step-by-step explanation:
Given parameters:
Mass of HCl = 13.5g
Unknown:
Mass of water produced = ?
Solution:
The reaction equation is shown below;
2HCl + CaCO₃ → CaCl₂ + CO₂ + H₂O
To solve this problem, let us find the number of moles of the given specie first then we can use that to find the mass of the unknown water.
Number of moles =

Molar mass of HCl = 1 + 35.5 = 36.5g/mol
Number of moles of HCl =
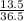 = 0.37mol
= 0.37mol
From the balanced chemical reaction;
2 mole of HCl will produce 1 mole of H₂O
0.37mole of HCl will produce
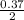 = 0.19mole of H₂O
= 0.19mole of H₂O
So to find the mass of H₂O;
Mass = Number of moles x molar mass
Molar mass of H₂O = 2(1) + 16 = 18g/mol
Mass of H₂O = 0.19 x 18 =3.3g