Each isotope of Oxygen has a different number of neutrons
Further explanation
The elements in nature have several types of isotopes
Atomic mass is the average atomic mass of all its isotopes
Isotopes are atoms has the same number of protons but has a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Some of the isotopes of oxygen are:
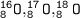
Each isotope has 8 protons and 8 electrons but has a different number of neutrons
For O-16: number of neutrons = 16-8 = 8
For O-17: number of neutrons = 17-8 = 9
For O-18: number of neutrons = 18-8 = 10