Answer:
326.56K
Explanation:
Step one:
Given
V1=0.45L
P1=2.3atm
T1=60 °
V2=0.6L
P2=1.54atm
T2=?
Step two:
Required:
T2
Applying the partial gas formula
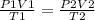
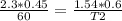
cross multiply we have
T2=(1.54*0.6*60)/2.3*0.45
T2=55.44/1.035
T2=53.56 °
in kelvin, we need to add 273
=273+53.56=326.56K
T2=326.56K
The new temperature of this gas (in K) is 326.56K