Answer:
0.15atm
Step-by-step explanation:
Given parameters:
Mass of dry ice = 1.28g
Volume of chamber = 5L
Temperature = 35.1°C = 35.1 + 273 = 308.1K
Unknown:
Pressure in the chamber = ?
Solution:
As we assume ideality for the gases in the cylinder, we use the equation below to solve the problem;
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹k⁻¹
T is the temperature
Let us find the number of moles;
Molar mass of CO₂ = 12 + 2(16) = 44g/mol
Number of moles of CO₂ =

Number of moles of CO₂ =
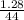 = 0.03moles
= 0.03moles
So;
Input the parameters and solve;
P x 5 = 0.03 x 0.082 x 308.1
5P = 0.73
P =
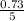 = 0.15atm
= 0.15atm