The limiting reactant : Na₂S
Further explanation
Reaction
Cu(NO₃)₂ (aq) + Na₂S (aq) ⇒ 2 NaNO₃ (aq) + CuS (s)
mol Cu(NO₃)₂ :(MW 187,56 g/mol)
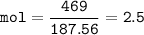
mol Na₂S :(MW 78,0452 g/mol)
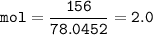
Limiting reactant(smaller ratio⇒ between mol and coefficient) :
1 mol Cu(NO₃)₂ : 1 mol Na₂S , so the limiting reactant : Na₂S( 2 < 2.5)