Answer:
a. [Ar]4s13d10.
Step-by-step explanation:
Hello!
In this case, since the electron configuration allows us to arrange the electrons moving around the atom of an element and equal the atomic number, we can write the electron configuration of copper as shown below:
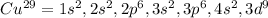
However, in terms of the argon's electron configuration, which is:
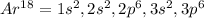
We infer that the electron configuration of copper in terms of that of argon's is:
![Cu^(29)=[Ar]4s^2,3d^9](https://img.qammunity.org/2021/formulas/chemistry/college/p9cuc466bctvwoaoyg5k9gwypgviuct680.png)
Therefore, the answer is a. [Ar]4s13d10 because the 4s² can be written as 4s¹ and therefore the 3d⁹ is changed to 3d¹⁰.
Best regards!