Answer:
4.2x10⁻⁴ M or 0.032 g/L.
Step-by-step explanation:
Hello!
In this case, for solubility product problems, we apply the concepts of equilibrium because an insoluble salt is ionized until a certain point limited by the solubility product constant, Ksp. Thus, we first write the ionization reaction of aluminum hydroxide at equilibrium:
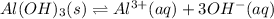
Next, we write the corresponding equilibrium expression:
![Ksp=[Al^(3+)][OH^-]^3](https://img.qammunity.org/2021/formulas/chemistry/college/6vvtkrvb88eblq9uaj1m88idjn3kqko9n0.png)
Which in terms of
 , the reaction extent, is:
, the reaction extent, is:
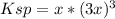
Because
 also represents the molar solubility of aluminum hydroxide at the considered temperature; now, we can write:
also represents the molar solubility of aluminum hydroxide at the considered temperature; now, we can write:
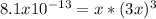
Which can be solved for x as follows:
![x=\sqrt[4]{(8.1x10^(-13))/(27) } \\\\x=4.2x10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/v48bskb235eit723kf4jfqewb5d1ay0quy.png)
Thus, the solubility is 4.2x10⁻⁴ M or mol/L and in g/L we have:
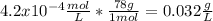
Best regards!