Answer:
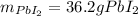
Step-by-step explanation:
Hello!
In this case, since the chemical reaction that takes place when Pb(NO3)2 and NaI are mixed is:
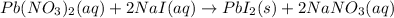
By which a solid precipitate of PbI2 is produced. In such a way, since we know the volume and molarity of NaI, we can compute the moles of NaI as shown below:
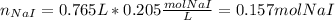
Next, since it is in a 2:1 mole ratio with PbI2 (molar mass = 461.01 g/mol) we compute the mass of PbI2 precipitate as shown below:
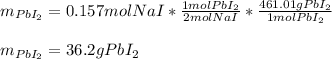
Best regards!