Solution :
In the process to isolate gold that has a 80 percent yield, a 3.00 g of Au is being isolated.
That is, the actual yield of Au is 3. 00 g
Therefore, we need to find the theoretical yield.
As we know,
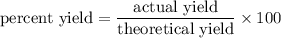
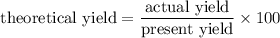
As actual yield = 3.00 g
percent yield = 80 %
So, theoretical yield =
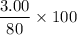
= 3.75 g
Thus he should be able to get 3.75 g which is the theoretical yield of Au.