Answer:
0.93g/mL
Step-by-step explanation:
Given parameters
Mass of wood = 3.16g
Initial volume of water = 20mL
Volume of water + wood = 23.4mL
Unknown:
Density of the wood = ?
Solution:
Density is the mass per volume of a substance.
It is mathematically expressed as;
Density =

Volume of wood = 23.4mL - 20mL = 3.4mL
So,
Density =
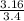 = 0.93g/mL
= 0.93g/mL