Answer:
a. K = [H+][NO2-]/[HNO2]
Step-by-step explanation:
The computation of the expression of the equilibrium constant is shown below:
Given that the weak acid is HNO_2 that exist in the solution that aqueous
The dissociation equation of
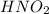
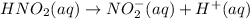
Now
Acidionization constant i.e.
![k_a = ([NO_(2)^(-)][H^(+)])/(HNO_(2))](https://img.qammunity.org/2021/formulas/chemistry/college/v1xdmlcfwuq0u3xmp51jihfdh52onmfkh3.png)
Therefore the correct option is a.
Hence, the same is to be considered