Answer:
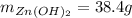
Step-by-step explanation:
Hello!
In this case, for the undergoing chemical reaction:
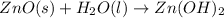
We evaluate the yielded moles of zinc hydroxide by each reactant as shown below:
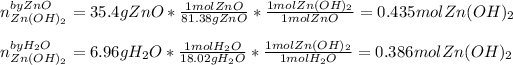
In such a way, since the water yields a smaller amount of zinc hydroxide we conclude it is the limiting reactant so the maximum mass is computed below:
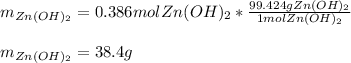
Because the water limits the yielded amount of zinc hydroxide.
Best regards!