Solution :
Given :
Dry bulb temperature, T = 30 degree C
Absolute pressure, P = 102 kPa
Partial pressure of water vapor,
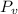 = 1.5 kPa
= 1.5 kPa
a). Relative humidity is given by
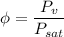
At T = 30 degree C, from the steam table, we have saturation temperature as 4.2469 kPa.
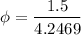
= 0.35319
= 35.31 %
b). Humidity ratio,
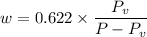
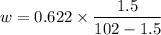
w = 0.009283 kg per kg of dry air
c). Dew point temperature is the saturation temperature at vapor pressure of air (can be found from steam table)
At
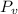 = 1.5 kPa, saturation temperature = 1.02 degree C
= 1.5 kPa, saturation temperature = 1.02 degree C
∴ Dew point temperature = 13.02 °C
d). Humidity ratio can be be defined as the ratio of mass of water vapor to the mass of dry air.
i.e.
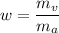
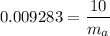
∴
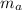 = 1077.170 kg of dry air
= 1077.170 kg of dry air