Answer:
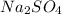
Step-by-step explanation:
In the case when there are more ions present in the solution so that solution contains less vapour pressure, high boiling point and less freezing point
Given that
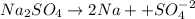
Now the ions present would be
= 2.0 × 3
= 6 ions
These ions represent the greater among all the solutions available
Therefore the
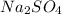 is the right answer
is the right answer
The same is to be considered