Answer:
196.07 m, 2.85 m and 0.15 m
Step-by-step explanation:
Frequency of AM radio signal, f₁ = 1530 kHz
Its wavelength can be given by :
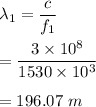
Frequency of FM radio signal, f₂ = 105.1 MHz
Its wavelength can be given by :
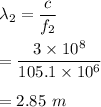
Frequency of cell phone signal, f₃ = 1.9 GHz
Its wavelength can be given by :
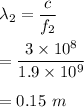
Hence, the required wavelengths are 196.07 m, 2.85 m and 0.15 m respectively.