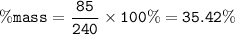The mass percent for a solution : 35.42%
Further explanation
The concentration of a substance can be expressed in several quantities such as moles, percent (%) weight/volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
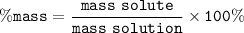
mass solute = 85 g
mass solution = 85 + 155 = 240 g