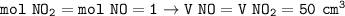a. 0.5 mol
b. 25 cm³
c. (i) 75 cm³
(ii) 50 cm³
Further explanation
Avogadro's hypothesis:
In the same T,P and V, the gas contains the same number of molecules
So the ratio of gas volume will be equal to the ratio of gas moles
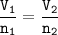
Reaction
2NO(g)+O₂(g)⇒2NO₂(g)
a. mol O₂
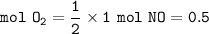
b. volume of O₂
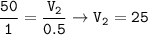
c. (i)total volume
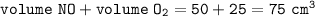
(ii)volume of NO₂